Abstract
Background: Standard therapies for newly diagnosed multiple myeloma (NDMM) patients who are ineligible for autologous stem-cell transplantation (ASCT) have been lenalidomide-dexamethasone (Rd), bortezomib-lenalidomide-dexamethasone (VRd) or bortezomib-melphalan-prednisone (VMP). The benefit of adding an anti-CD38 monoclonal antibody (mAb) to the standard treatments of VMP and Rd has been demonstrated in two large phase 3 studies (1, 2). Isatuximab is an anti-CD38 mAb approved in combination with pomalidomide-dexamethasone and carfilzomib-dexamethasone to treat relapsed/refractory MM, and has also shown clinical responses as combination therapy to treat NDMM (3).
Corticosteroids have been the backbone of most myeloma targeted therapies since the discovery of their effectiveness in 1962. Due to the continuous treatment paradigm, the cumulative dose of corticosteroids in patients is substantial. Steroids inflict reduction in both short- and long-term quality of life and increase patients' receptiveness for infections. Infections are one of the main reasons for complications and death during active first line treatment, and the rate rises with age. Both steroid-free regimens and regimens with reduced corticosteroids have demonstrated improved safety and similar efficacy as regimens containing steroids.
Aim: The study will evaluate isatuximab (Isa) in combination with bortezomib (V) and lenalidomide (R) with minimal dexamethasone (d) as first-line treatment in transplant-ineligible patients. The primary endpoint is the number of patients who achieve measurable residual disease negative (Euroflow NGF 10-5) complete response during and/or after 18 cycles of study treatment. Secondary endpoints include progression free survival, overall survival, overall response rate, safety evaluations and patient-reported outcome.
Methods: The REST study is an academic, single arm, open-label, phase 2 study of NDMM patients ineligible for ASCT. 50 patients will be included and receive Isa-VRd (Isa:10 mg/kg IV Days 1, 8, 15, 22 during cycle 1, Q2W cycle 2-18; V: 1,3 mg/m2 SC Days 1,8,15 during cycle 1-8; R: 25 mg PO Days 1-21 during all cycles; d: 20 mg PO Days 1,8,15,22 only for the first 2 cycles), all 28-day cycles.
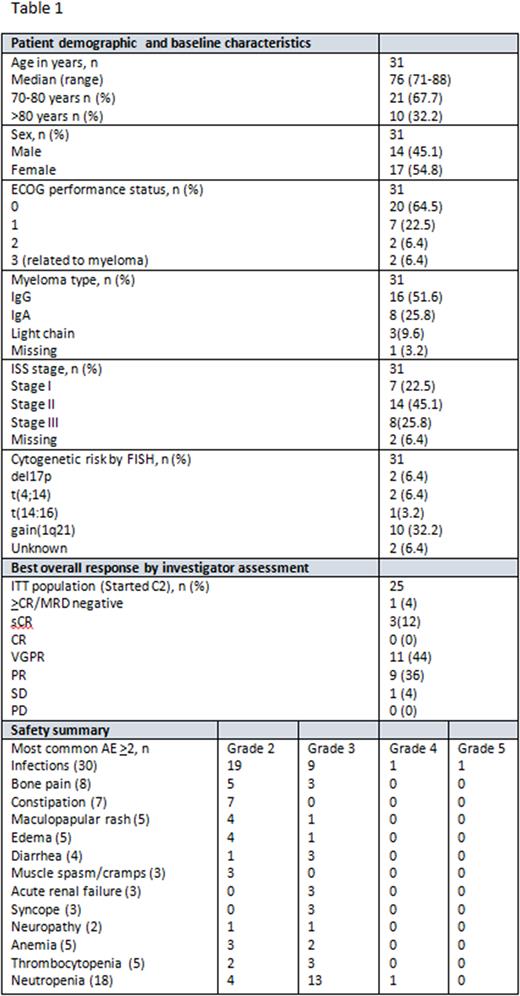
Results: As of July 7, 2022, 31 patients have been enrolled. Baseline characteristics, safety summary and preliminary results can be found in Table 1. The median number of cycles started by patients was 7 (range 1-13) and the median duration of exposure was 28 (range 1-52) weeks. 17 patients developed 30 grade >3 non-hematological adverse events (AE), including infections (n=12), skeletal pain (n=3), acute renal failure (n=3), diarrhea (n=3), syncope (n=3), peripheral sensory neuropathy (n=1), edema (n=1), cerebral insult (n=1), lung embolus (n=1), rash (n=1) and opiate intoxication (n=1). One patient has discontinued the study due to death from septicemia with staphylococcus aureus. At a median follow up of 7 months, the ORR was 96% (24/25) and the >very good partial response rate was 60% (15/25 patients).
Conclusions: Isa-VRd in the transplant ineligible population has a tolerable safety profile. Isa-VRd shows encouraging preliminary efficacy in NDMM patients who are ineligible for transplant. Enrollment and follow-up is ongoing.
Acknowledgements: This research was funded by Sanofi.
1. Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med. 2019;380(22):2104-15.
2. Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378(6):518-28.
3. Trudel SJTL. Incorporating isatuximab in the treatment of multiple myeloma. 2019;394(10214):2045-7.
Disclosures
Askeland:Amgen: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Sanofi: Consultancy, Research Funding. Slørdahl:Takeda: Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Bristil Myers Squibb: Consultancy; GSK: Consultancy, Honoraria. Hermansen:Sanofi: Honoraria; Bristol Myers Squibb: Honoraria; Janssen-Cilag: Honoraria, Other: Advisory board participation; Amgen: Honoraria; Takeda: Honoraria, Other: Conference participant support. Schjesvold:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Skylite DX: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Targovax: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal